About the lab
We are a research group at the Division of Molecular Metabolism at the Karolinska Institute studying how mitochondrial metabolism and mitochondrial gene expression shape tissue function in health and disease. By combining genetic models (Drosophila and mouse), patient-derived cells, and multi-omics approaches, we aim to define causal mechanisms of mitochondrial dysfunction and translate them into improved diagnostics and precision medicine for inherited metabolic disorders. We work closely with the Centre for Inherited Metabolic Diseases at Karolinska University Hospital, integrating clinical insight with mechanistic biology.
Anna Wredenberg is an MD at the Centre for Inherited Metabolic Diseases at the Karolinska University Hospital, which serves patients from across Sweden and provides comprehensive molecular, bioenergetic, and metabolic diagnostics. Rapid advances in genomic and multi-omics technologies now make it possible to connect patient phenotypes to causal molecular mechanisms with unprecedented resolution. Through close collaboration with the clinic, we combine clinical insight with experimental biology to validate disease mechanisms and advance precision diagnostics for inherited metabolic disorders.
Research
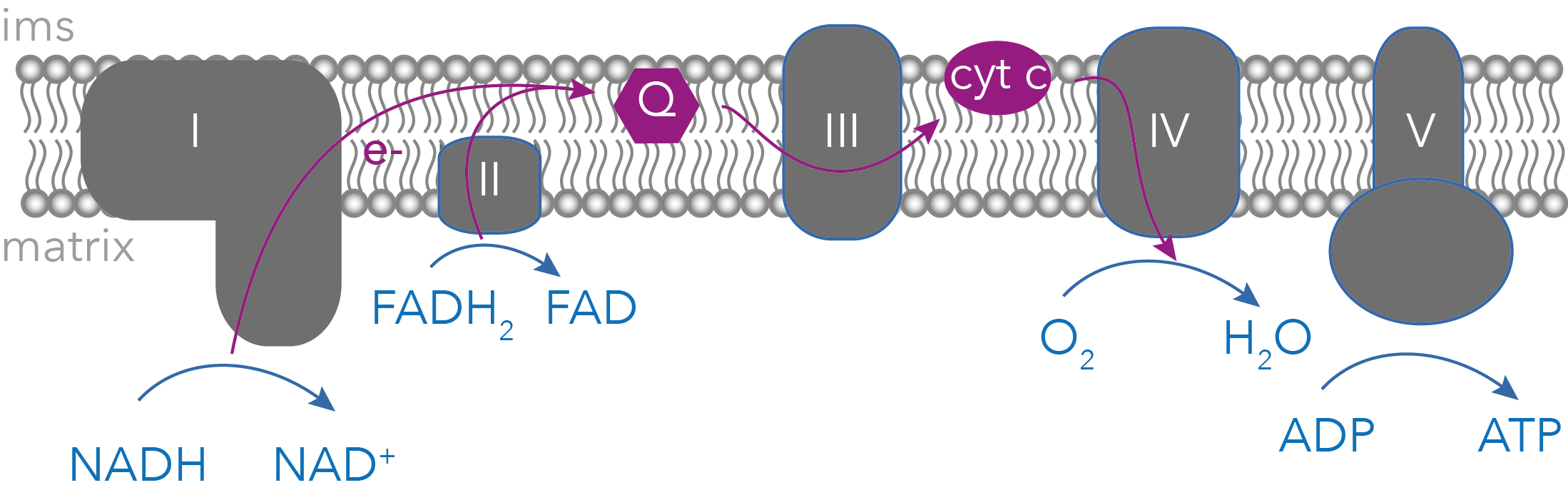
Mitochondria form a highly dynamic network in almost every eukaryotic cell, adapting rapidly to changing cellular demands. While best known for ATP production, mitochondria also control essential metabolic and signalling pathways, including lipid metabolism, iron–sulfur cluster biogenesis, calcium homeostasis, redox balance, and cell death. Because of this central role, mitochondrial dysfunction contributes to diverse human diseases, including neurodegeneration, heart disease, and diabetes, and is linked to ageing.
Our research aims to define how mitochondrial dysfunction reshapes cellular metabolism and drives disease. A major focus is on mitochondrial methylation potential and how it influences mitochondrial function, one-carbon metabolism, and cell physiology. We also study how mitochondrial gene expression is regulated post-transcription, with particular emphasis on mitochondrial RNA turnover and transcript stability.
We combine genetic model systems, including patient-derived material, Drosophila, and mouse models, with molecular biology and multi-omics approaches. By integrating proteomic and metabolomic data, we map mitochondrial pathways and protein networks and determine how specific metabolic constraints translate into cellular dysfunction. Through close collaboration with the clinic, we connect these mechanisms to patient biology and inherited metabolic disease.
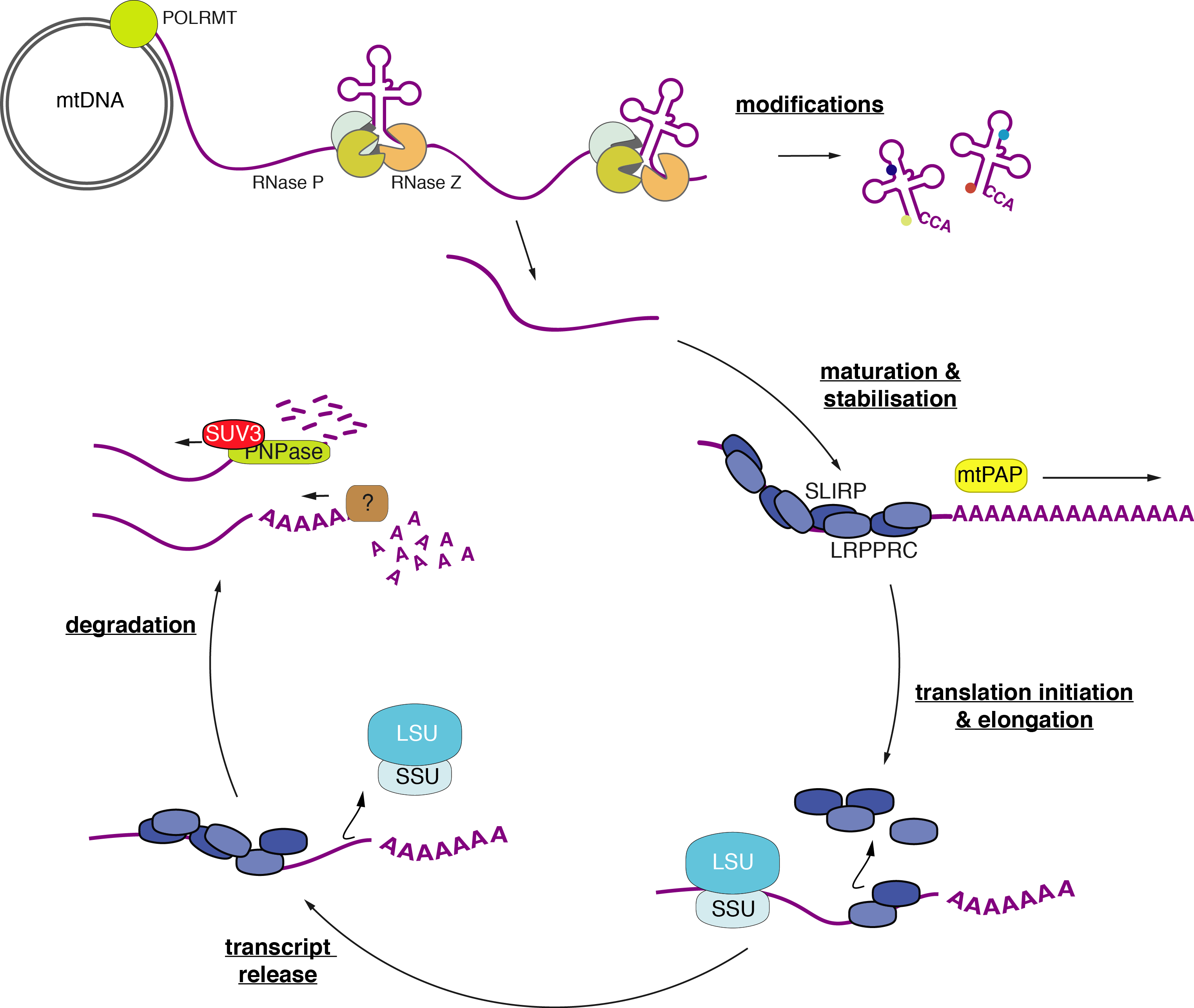
Mitochondria contain their own DNA, which is transcribed and translated within the organelle. Despite major advances, the regulation of mitochondrial RNA processing, modification, translation, and turnover remains poorly understood. We use genetically modified Drosophila and mammalian models to define the mechanisms that govern mitochondrial RNA metabolism and its coupling to mitochondrial translation.
Mitochondrial gene expression begins with long polycistronic transcripts that require precise processing to generate functional RNAs. While the tRNA punctuation model explains cleavage at tRNA boundaries, many mitochondrial transcripts lack flanking tRNAs and require alternative processing routes. We discovered that cleavage of these non-canonical transcripts produces 3′-terminal phosphate groups, which are removed by the mitochondrial phosphatase ANGEL2, and we proposed that FASTKD family proteins are key factors in this pathway.
Mitochondrial dysfunction causes rare inborn errors of metabolism (IEM) and contributes to common diseases, including cancer, heart failure, neurodegeneration, diabetes, and ageing. Although genomic sequencing has transformed diagnostics, many patients still lack functional validation of candidate variants. In close collaboration with the Centre for Inherited Metabolic Diseases at Karolinska University Hospital, we validate novel gene variants and define their molecular consequences.
We use complementary experimental systems, including patient-derived iPS cell models and mutation-specific Drosophila models, combined with bioenergetic assays and multi-omics analyses. By mapping proteomic and metabolic changes across patients and models, we aim to build co-dependency networks that reveal how mitochondrial defects propagate through cellular metabolism. We are also exploring whether untargeted proteomics can support diagnosis in patients with mitochondrial dysfunction of unknown cause.
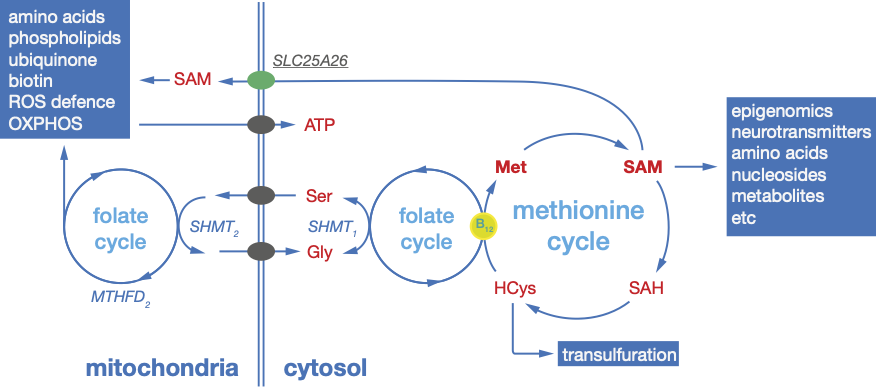
Mitochondria sit at the centre of cellular metabolism, interacting with pathways that provide both energy and essential metabolites. One-carbon metabolism is a major source of methyl groups and has emerged as an early indicator of mitochondrial dysfunction. Within mitochondria, methylation of key metabolic proteins links one-carbon metabolism directly to energy conversion.
We combine genetic models with proteomics, metabolomics, and molecular biology to understand how mitochondrial methylation potential regulates mitochondrial function, gene expression, and cellular metabolism. We generated mitochondrial methylproteomes in flies, mice, and humans and found evidence that many methylation events occur before mitochondrial protein import. Our work also connects one-carbon metabolism and bioenergetics through the regulation of cytosolic and mitochondrial SAM levels. We are currently defining how these mechanisms shape metabolism in different tissues and disease contexts.
PI

MD, PhD
2007 PhD Karolinska Institutet
2009 Swedish Medical Licence
2010 – 2012 PostDoc Max-Planck Institute for Biology of Ageing
2019 – Consultant in Clinical Genetics | Karolinska University Hospital
2012 – Research Group Leader | Karolinska Institutet
2024 – Professor of Mitochondrial Biology
Anna.Wredenberg@ki.se

PhD
2004 PhD University of Newcastle
2004 – 2008 PostDoc Karolinska Institutet
2008 – 2012 PostDoc Max-Planck Institute for Biology of Ageing
2012 – Karolinska University Hospital & Karolinska Institutet
PostDocs




PhD
2024 PhD Institute of Cancer Research | London
2025 PostDoc Karolinska Institutet


PhD
2021 PhD Univ. of Cologne, Germany
2021- PostDoc Karolinska Institutet
Students and affiliations



MSc
2024 MSc Imperial College London
2024 Research Assistant Karolinska Institutet
2025 PhD student
Alumni
2012 – 2013 Sarah Hempenstall – PostDoc | Leiden University Medical Center | The Netherlands
2014 – 2016 Olle Dahlberg – Scientist | Karolinska University hospital | Sweden
2014 – 2018 Aleksandra Pajak – Senior Laboratory Research Scientist | The Francis Crick Institute | UK
2013 – 2021 Javier Calvo-Garrido | Scientific Advisor | Laboratorios Rovi | Madrid | Spain
2017 – 2021 David Moore | Associate Consultant | Oxford Pharmagenesis | UK
2018 – 2022 Marco Moedas | Clinical Chemist | CMMS | Karolinska University Hospital | Sweden
2012 – 2022 Paula Clemente | Junior PI | Universidad Autónoma de Madrid | Spain
2014 – 2019 Camilla Maffezzini | PostDoc | Stem Cell and Neurogenesis Unit | San Raffaele Scientific Institute | Italy
2016 – 2021 Florian Schober | PostDoc | Max-Planck Institute for Biochemistry | Martinsried | Germany
2014 – 2022 Isabelle Laine | Clinical Training | Region Stockholm | Sweden
2016 – 2025 Helene Bruhn | Clinical Geneticist | CMMS | Karolinska University Hospital | Sweden
Publications
Wibom R, Alsina D, Naess K, Engvall M, Freyer C, Wedell A, Wredenberg A. (2026) Advancing a sensitive method for measuring mitochondrial ATP production in small muscle biopsy samples. Analytical Biochemistry 709:116004 LINK
Sörensen L, Asin-Cayuela J, Barbaro M, Bruhn H, Engvall M, Lesko N, Naess K, Oscarson M, Shen Y, Ueberschär M, Wredenberg A, Sterky FH, Wedell A, Zetterström RH. (2025) Next-Generation Sequencing in the Diagnostic Workup of Neonatal Dried Blood Spot Screening in Sweden 2015-2023 Int J Neonatal Screen 11(3):73 LINK
Glasgow RIC, Singh V, Peña-Pérez L, Wilhalm A, Moedas MF, Moore D, Rosenberger FA, Li X, Atanassov I, Saba M, Cipullo M, Rorbach J, Wedell A, Freyer C, Amunts A, Wredenberg A. (2025) The mitochondrial methylation potential gates mitoribosome assembly. Nature Communications 16(1):5388 LINK
El Fissi N, Rosenberger FA, Chang K, Wilhalm A, Barton-Owen T, Hansen FM, Golder Z, Alsina D, Wedell A, Mann M, Chinnery PF, Freyer C, Wredenberg A. (2024) Preventing excessive autophagy protects from the pathology of mtDNA mutations in Drosophila melanogaster. Nature Communications. 15(1):10719 LINK
Correia SP, Moedas MF, Taylor LS, Naess K, Lim AZ, McFarland R, Kazior Z, Rumyantseva A, Wibom R, Engvall M, Bruhn H, Lesko N, Végvári Á, Käll L, Trost M, Alston CL, Freyer C, Taylor RW, Wedell A, Wredenberg A. (2024) Quantitative proteomics of patient fibroblasts reveal biomarkers and diagnostic signatures of mitochondrial disease JCI Insight. 9(20):e178645. LINK
Rogal J, Zamproni LN, Nikolakopoulou P, Ygberg S, Wedell A, Wredenberg A, Herland A. (2024) Human In Vitro Models of Neuroenergetics and Neurometabolic Disturbances: Current Advances and Clinical Perspectives. Stem Cells Transl Med. 2024 Apr 8:szae021. (review) LINK
Bruhn H, Naess K, Ygberg S, Peña-Pérez L, Lesko N, Wibom R, Freyer C, Stranneheim H, Wedell A, Wredenberg A. (2024). Novel Synonymous and Deep Intronic Variants Causing Primary and Secondary Pyruvate Dehydrogenase Complex Deficiency. Human Mutation. Article ID 1611838 LINK
Filograna R, Gerlach J, Choi HN, Rigoni G, Barbaro M, Oscarson M, Lee S, Tiklova K, Ringnér M, Koolmeister C, Wibom R, Riggare S, Nennesmo I, Perlmann T, Wredenberg A, Wedell A, Motori E, Svenningsson P, Larsson NG. (2024) PARKIN is not required to sustain OXPHOS function in adult mammalian tissues. NPJ Parkinsons Dis. 10(1):93 LINK
Vuckovic A, Freyer C, Wredenberg A#, Hillen H#. (2024) The molecular machinery for maturation of primary mtDNA transcripts. Human Molecular Genetics (review) LINK
Ben-Shabat I, Kvarnung M, Sperker W, Bruhn H, Wredenberg A, Wibom R, Nennesmo I, Engvall M, Paucar M. (2023) Ataxia Syndrome With Hearing Loss and Nephronophthisis Associated With a Novel Homozygous Variant in XPNPEP3. Neurol Genet. 9(6):e200100. LINK
Zhang J, Koolmeister C, Han J, Filograna R, Hanke L, Àdori M, Sheward DJ, Teifel S, Gopalakrishna S, Shao Q, Liu Y, Zhu K, Harris RA, McInerney G, Murrell B, Aoun M, Bäckdahl L, Holmdahl R, Pekalski M, Wedell A, Engvall M, Wredenberg A, Karlsson Hedestam GB, Castro Dopico X, Rorbach J. (2023) Antigen receptor stimulation induces purifying selection against pathogenic mitochondrial tRNA mutations. JCI Insight 8(17):e167656 LINK
Erdinc D, Macao B, Valenzuela S, Lesko N, Naess K, Peter B, Bruhn H, Wedell A, Wredenberg A#, Falkenberg M#. (2023) The disease-causing mutation p.F907I reveals a novel pathogenic mechanism for POLγ-related diseases. Biochim Biophys Acta Mol Basis Dis. 1869(7):166786. LINK
Mattison KA, Tossing G, Mulroe F, Simmons C, Butler KM, Schreiber A, Alsadah A, Neilson DE, Naess K, Wedell A, Wredenberg A, Sorlin A, McCann E, Burghel GJ, et al. (2023) ATP6V0C variants impair V-ATPase function causing a neuro-developmental disorder often associated with epilepsy. Brain 146(4):1357-1372 LINK
Vaz R, Wincent J, Elfissi N, Rosengren Forsblad K, Pettersson M, Naess K, Wedell A, Wredenberg A, Lindstrand A, Ygberg S. (2022) A Missense Variant in PDK1 Associated with Severe Neurodevelopmental Delay and Epilepsy. Biomedicines. 10(12):3171. LINK
Clemente P#, Calvo-Garrido J, Pearce SF, Schober FA, Shigematsu M, Siira SJ, Laine I, Spåhr H, Steinmetzger C, Petzold K, Kirino Y, Wibom R, Packham O, Filipovska A, Rorbach J, Freyer C#, Wrednebrg A#. (2022) ANGEL2 phosphatase activity is required for non-canonical mitochondrial RNA processing. Nat Commun 13, 5750 LINK
Misic J, Milenkovic D, Al-Behadili A, Xie X, Jiang M, Jiang S, Filograna R, Koolmeister C, Siira SJ, Jenninger L, Filipovska A, Clausen AR, Caporali L, Valentino ML, La Morgia C, Carelli V, Nicholls TJ, Wredenberg A, Falkenberg M, Larsson NG. (2022) Mammalian RNase H1 directs RNA primer formation for mtDNA replication initiation and is also necessary for mtDNA replication completion. Nucleic Acids Res. 50(15):8749-8766 LINK
Rosenhahn E*, O’Brien TJ*, Zaki MS, Sorge I, Wieczorek D, Rostasy K, Vitobello A, Nambot S, Alkuraya FS, Hashem MO, et al. (2022) Bi-allelic loss-of-function variants in PPFIBP1 cause a neurodevelopmental disorder with microcephaly, epilepsy, and periventricular calcifications. Am J Hum Genet. 109(8):1421-1435 LINK
Schober FA*, Tang JX*, Sergeant K*, Moedas MF, Zierz CM, Moore D, Smith C, Lewis D, Guha N, Hopton S, Falkous G, Lam A, Pyle A, Poulton J, Gorman GS, Taylor RW, Freyer C#, Wredenberg A#. (2022) Pathogenic SLC25A26 variants impair SAH transport activity causing mitochondrial disease. Human Molecular Genetics, ddac002
Stödberg, T.#, Magnusson, M., Lesko, N., Wredenberg, A., Martin Munoz, D., Stranneheim, H., Wedell, A.# (2020) SLC12A2 mutations cause NKCC1 deficiency with encephalopathy and impaired secretory epithelia. Neurology Genetics 6(4): e478 LINK
Alsina, D.*, Lytovchenko, O.*, Schab, A., Atanassov, I., Schober, FA., Jiang, M., Koolmeister, C., Wedell, A., Taylor, RW., Wredenberg, A., Larsson, N-G. (2020) FBXL 4 deficiency increases mitochondrial removal by autophagy. EMBO Mol Med. e11659 LINK
Maffezzini, C., Calvo-Garrido, J, Wredenberg, A.#, & Freyer, C.# (2020) Metabolic regulation of neurodifferentiation in the adult brain. Cellular and Molecular Life Sciences. LINK (Review)
Gopalakrishna, S.*, Pearce, S.F.*, Dinan, A.M., Schober, F.A., Cipullo, M., Spåhr, H., Khawaja, A., Maffezzini, C., Freyer, C., Wredenberg, A., Atanassov, I., Firth, A.E., Rorbach, J. (2019). C6orf203 is an RNA-binding protein involved in mitochondrial protein synthesis. Nucleic Acids Research. 47(17): 9386–9399 LINK
Javier Calvo-Garrido J, Winn D, Maffezzini C, Wedell A, Freyer C, Falk A, Wredenberg A. (2021) Protocol for the derivation, culturing, and differentiation of human iPS-cell-derived neuroepithelial stem cells to study neural differentiation in vitro. STAR Protocols, 2(2):100528 LINK
Ferreira, CR, Rahman S, Keller M, Zschocke J, ICIMD Advisory Group (2021) An international classification of inherited metabolic disorders (ICIMD). Journal of Inherited Metabolic Diseases, Vol. 44(1):164-177 LINK
Engvall M, Kawasaki A, Carelli V, Wibom R, Bruhn H, Lesko N, Schober FA, Wredenberg A, Wedell A, and Traisk F. (2021) Case Report: A Novel Mutation in the Mitochondrial MT-ND5 Gene Is Associated With Leber Hereditary Optic Neuropathy (LHON). Frontiers in Neurology, Vol. 12:652590 LINK
Stranneheim, H., Lagerstedt-Robinson, K., Magnusson, M., Kvarnung, M., Nilsson, D., Lesko, N., Engvall, M., Anderlid, B-M., Arnell, H. et al. (2021) Integration of whole genome sequencing into a health care setting: High diagnostic rates across multiple clinical entities in 3219 rare disease patients. Genome Medicine, Vol. 13:40 LINK
Schober, FA.*, Moore, M.*, Atanassov, I., Moedas, MF., Clemente, P., Végvári, Á., El Fissi, N., Filograna, R., Bucher, A-L., Hinze, Y., The, M., Hedman, E., Chernogubova, E., Begzati, A., Wibom, R., Jain, M., Nilsson, R., Käll, L., Wedell, A., Freyer, C.#, and Wredenberg, A.#. (2021) The One-Carbon Pool Controls Mitochondrial Energy Metabolism via Complex I and Iron-Sulfur Clusters. Science Advances Vol. 7, no. 8, eabf0717. LINK
Schober, FA.*, Atanassov, I.*, Moore, M., Calvo-Garrido, J., Moedas, MF., Wedell, A., Freyer, C.#, Wredenberg, A.# (2021) Direct proteome labelling in fruit flies with SILAF reveals differential phosphorylation of mitochondrial proteins upon loss of OXPHOS subunits- Molecular & Cellular Proteomics, Vol. 20:100065 LINK
Schober FA, Atanassov I, Freyer C, Wredenberg A. (2020) Quantitative Proteomics in Drosophila with Holidic Stable-Isotope Labeling of Amino Acids in Fruit Flies (SILAF). In: Minczuk M., Rorbach J. (eds) Mitochondrial Gene Expression. Methods in Molecular Biology, Vol. 2192:75-87 LINK
Correia SP*, Moedas MF*, Naess K, Bruhn H, Maffezzini C, Calvo-Garrido J, Lesko N, Wibom R, Schober FA, Jemt A, Stranneheim H, Freyer C, Wedell A#, Wredenberg A#. (2021) Severe congenital lactic acidosis and hypertrophic cardiomyopathy caused by an intronic variant in NDUFB7. Human Mutation, Vol. 42(2):278-384 LINK
Bruhn H, Samuelsson K, Schober FA, Engvall M, Lesko N, Wibom R, Nennesmo I, Calvo-Garrido J, Press R, Stranneheim H, Freyer C, Wedell A, Wredenberg A. (2021) A novel mutation m.10372A>G in MT-ND3 causing sensorimotor axonal polyneuropathy. Neurology: Genetics, Vol. 7(2):e566 LINK
Cipullo, M., Pearce, SF., Lopez Sanchez, IG., Gopalakrishna, S., Krüger, A., Schober, FA., Busch, JD., Li, X., Wredenberg, A., Atanassov, I., Rorbach, J. (2021) Human GTPBP5 is involved in the late stage of mitoribosome large subunit assembly. Nucleic Acids Res. 49(1):354-370. LINK
Naess K, Bruhn H, Stranneheim H, Freyer C, Wibom R, Mourier A, Engvall M, Nennesmo I, Lesko N, Wredenberg A, Wedell A, von Döbeln U. (2021) Clinical Presentation, Genetic Etiology and Coenzyme Q10 Level in 55 Children with Combined Enzyme Deficiencies of the Mitochondrial Respiratory Chain. The Journal of Pediatrics. vol 228:240-251.e2
LINK
Pajak, A.*, Laine, I.*, Clemente, P., El-Fissi, N., Schober, F.A., Maffezzini, C., Calvo-Garrido, J., Wibom, R., Filograna, R., Dhir, A., Wedell, A., Freyer, C.#, Wredenberg, A.#. (2019). Defects of mitochondrial RNA turnover lead to the accumulation of double-stranded RNA in vivo. PLoS Genetics, 15(7): e1008240 LINK
Schober, F.A.*, Atanassov, I.*#, Moore, D., Wedell, A., Freyer, C.#, Wredenberg, A.# (2019) Versatile proteome labelling in fruit flies with SILAF. Preprint at: BioRxiv LINK
Maffezzini, C.*, Laine, I.*, Dallabona, C., Clemente, P., Calvo-Garrido, J., Wibom, R., Naess, K., Barbaro, M., Falk, A., Donnini, C., Freyer, C.#, Wredenberg, A.#, Wedell, A.# (2019) Mutations in the mitochondrial tryptophanyl-tRNA synthetase cause growth retardation and progressive leukoencephalopathy. Molecular Genetics & Genomic Medicine, 7(6)e653 LINK
Olivé, M.*, Engvall, M.*, Ravenscroft, G.*, Cabrera-Serrano, M., Jiao, H., Bortolotti, C.A., Pignataro, M., Lambrughi, M., Jiang, H., Forrest, A.R.R., et al. (2019). Myoglobinopathy is an adult-onset autosomal dominant myopathy with characteristic sarcoplasmic inclusions. Nature Communications, 10(1):1396 LINK
Calvo-Garrido, J.*, Maffezzini, C.*, Schober, F.A., Clemente, P., Uhlin, E., Kele, M., Stranneheim, H., Lesko, N., Bruhn, B., Svenningsson, P., Falk, A., Wedell, A., Freyer, C.#, Wredenberg, A.#. (2019). SQSTM1/p62-directed metabolic reprogramming is essential for normal neurodifferentiation. Stem Cell Reports, 12(4):p696-711 LINK
Filograna, R., Koolmeister, C., Upadhyay, M., Pajak, A., Clemente, P., Wibom, R., Simard, M.L., Wredenberg, A., Freyer, C., Stewart, J.B., Larsson, N.-G. (2019). Modulation of mtDNA copy number ameliorates the pathological consequences of a heteroplasmic mtDNA mutation in the mouse. Science Advances, 5(4):eaav9824 LINK
Katsu-Jiménez, Y., Vázquez-Calvo, C., Maffezzini, C., Halldin, M., Peng, X., Freyer, C., Wredenberg, A., Giménez-Cassina, A., Wedell, A., Arnér, E.S.J. (2019). Absence of TXNIP in humans leads to lactic acidosis and low serum methionine linked to deficient respiration on pyruvate. Diabetes, 68(4):709-723 LINK
Richter, U., Evans, M.E., Clark, W.C., Marttinen, P., Shoubridge, E.A., Suomalainen, A., Wredenberg, A., Wedell, A., Pan, T., Battersby, B.J. (2018) RNA modification landscape of the human mitochondrial tRNALys regulates protein synthesis. Nature Communications, 9(1):3966 LINK
Paucar, M., Pajak, A., Freyer, C., Bergendal, Å., Döry, M., Laffita-Mesa, J.M., Stranneheim, H., Lagerstedt-Robinson, K., Savitcheva, I., Walker, R.H., Wedell, A., Wredenberg, A., Svenningsson, P. (2018) Chorea, psychosis, acanthocytosis, and prolonged survival associated with ELAC2 mutations. Neurology, pii:10.1212/WNL.0000000000006320 LINK
Herebian, D., Seibt, A., Smits, S.H.J., Bünning, G., Freyer, C., Prokisch, H., Karall, D., Wredenberg, A., Wedell, A., López, L.C., Mayatepek, E., Distelmaier, F. (2017) Detection of 6-demethoxyubiquinone in CoQ10 deficiency disorders: Insights into enzyme interactions and identification of potential therapeutics. Mol Genet Metab. 2017 Jul;121(3):216-223. LINK
Siibak, T.*, Clemente, P.*, Bratic, A., Bruhn, H., Kauppila, T.E.S., Macao, B., Schober, F.A., Lesko, N., Wibom, R., Naess, K., Nennesmo, I., Wedell, A., Peter, B., Freyer, C., Falkenberg, M.#, Wredenberg, A.# (2017) A multi-systemic mitochondrial disorder due to a dominant p.Y955H disease variant in DNA polymerase gamma. Hum Mol Genet. 2017 Jul 1;26(13):2515-2525.
Tegelberg, S., Tomašić, N., Kallijärvi, J., Purhonen, J., Elmér, E., Lindberg, E., Nord, D.G., Soller, M., Lesko, N., Wedell, A., Bruhn, H., Freyer, C., Stranneheim, H., Wibom, R., Nennesmo, I., Wredenberg, A., Eklund, E.A., Fellman, V. (2017) Respiratory chain complex III deficiency due to mutated BCS1L: a novel phenotype with encephalomyopathy, partially phenocopied in a Bcs1l mutant mouse model. Orphanet J Rare Dis. 2017 Apr 20;12(1):73.
Haack TB, Ignatius E, Calvo-Garrido J, Iuso A, Isohanni P, Maffezzini C, Lönnqvist T, Suomalainen A, Gorza M, Kremer LS, Graf E, Hartig M, Berutti R, Paucar M, Svenningsson P, Stranneheim H, Brandberg G, Wedell A, Kurian MA, Hayflick SA, Venco P, Tiranti V, Strom TM, Dichgans M, Horvath R, Holinski-Feder E, Freyer C, Meitinger T, Prokisch H, Senderek J, Wredenberg A, Carroll CJ, Klopstock T. (2016) Absence of the Autophagy Adaptor SQSTM1/p62 Causes Childhood-Onset Neurodegeneration with Ataxia, Dystonia, and Gaze Palsy. Am J Hum Genet. 2016 Sep 1;99(3):735-743. LINK
Bratic A, Clemente P, Calvo-Garrido J, Maffezzini C, Felser A, Wibom R, Wedell A, Freyer C, Wredenberg A. (2016) Mitochondrial Polyadenylation Is a One-Step Process Required for mRNA Integrity and tRNA Maturation. PLoS Genet. 2016 May 13;12(5):e1006028.
Bratic A, Kauppila TE, Macao B, Grönke S, Siibak T, Stewart JB, Baggio F, Dols J, Partridge L, Falkenberg M, Wredenberg A, Larsson NG. (2015) Complementation between polymerase- and exonuclease-deficient mitochondrial DNA polymerase mutants in genomically engineered flies. Nat Commun. 2015 Nov 10;6:8808.
Kishita Y, Pajak A, Bolar NA, Marobbio CM, Maffezzini C, Miniero DV, Monné M, Kohda M, Stranneheim H, Murayama K, Naess K, Lesko N, Bruhn H, Mourier A, Wibom R, Nennesmo I, Jespers A, Govaert P, Ohtake A, Van Laer L, Loeys BL, Freyer C, Palmieri F, Wredenberg A, Okazaki Y, Wedell A. (2015) Intra-mitochondrial Methylation Deficiency Due to Mutations in SLC25A26. Am J Hum Genet. 2015 Nov 5;97(5):761-8.
Gineste C, Hernandez A, Ivarsson N, Cheng AJ, Naess K, Wibom R, Lesko N, Bruhn H, Wedell A, Freyer C, Zhang SJ, Carlström M, Lanner JT, Andersson DC, Bruton JD, Wredenberg A, Westerblad H. (2015) Cyclophilin D, a target for counteracting skeletal muscle dysfunction in mitochondrial myopathy. Hum Mol Genet. 2015 Dec 1;24(23):6580-7.
Clemente P, Pajak A, Laine I, Wibom R, Wedell A, Freyer C, Wredenberg A. (2015) SUV3 helicase is required for correct processing of mitochondrial transcripts. Nucleic Acids Res. 2015 Sep 3;43(15):7398-413.
Freyer C, Stranneheim H, Naess K, Mourier A, Felser A, Maffezzini C, Lesko N, Bruhn H, Engvall M, Wibom R, Barbaro M, Hinze Y, Magnusson M, Andeer R, Zetterström RH, von Döbeln U, Wredenberg A, Wedell A. (2015) Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid. J Med Genet. 2015 Nov;52(11):779-83.
Stranneheim H, Engvall M, Naess K, Lesko N, Larsson P, Dahlberg M, Andeer R, Wredenberg A, Freyer C, Barbaro M, Bruhn H, Emahazion T, Magnusson M, Wibom R, Zetterström RH, Wirta V, von Döbeln U, Wedell A. (2014) Rapid pulsed whole genome sequencing for comprehensive acute diagnostics of inborn errors of metabolism. BMC Genomics. 2014 Dec 11;15(1):1090.
Acuna-Hidalgo R, Schanze D, Kariminejad A, Nordgren A, Kariminejad MH, Conner P, Grigelioniene G, Nilsson D, Nordenskjöld M, Wedell A, Freyer C, Wredenberg A, Wieczorek D, Gillessen-Kaesbach G, Kayserili H, Elcioglu N, Ghaderi-Sohi S, Goodarzi P, Setayesh H, van de Vorst M, Steehouwer M, Pfundt R, Krabichler B, Curry C, MacKenzie MG, Boycott KM, Gilissen C, Janecke AR, Hoischen A, Zenker M. (2014) Neu-Laxova syndrome is a heterogeneous metabolic disorder caused by defects in enzymes of the L-serine biosynthesis pathway. Am J Hum Genet. 2014 Sep 4;95(3):285-93.
Wredenberg, A.*, Lagouge, M.*, Bratic, A.*, Metodiev, M.D., Spåhr, H., Mourier, A., Freyer, C., Ruzzenente, B., Tain, L., Grönke, S., Baggio, F., Kukat, C., Kremmer, E., Wibom, R., Polosa, P.L., Habermann, B., Partridge, L., Park, C.B., Larsson, N.-G. (2013) MTERF3 regulates mitochondrial ribosome biogenesis in invertebrates and mammals. PLoS Genet. 2013;9(1):e1003178. LINK
Freyer, C., Clemente, P. & Wredenberg, A. Mitochondrial RNA Turnover in Metazoa. in RNA Metabolism in Mitochondria (eds. Cruz-Reyes, J. & Gray, M. W.) 17–46 (Springer International Publishing, 2018). LINK
Hagström, E., Freyer, C., Battersby, B.J., Stewart, J.B., & Larsson, N.-G. (2013). No recombination of mtDNA after heteroplasmy. Nucleic Acids Research, 42(2): 1111-1116. LINK
Milenkovic, D., Matic, S., Kühl, I., Ruzzenente, B., Freyer, C., Jemt, E., et al. (2013). TWINKLE is an essential mitochon- drial helicase required for synthesis of nascent D-loop strands and complete mtDNA replication. Human Molecular Genetics, 22(10): 1983–1993. LINK
Ross, J.M.*, Stewart, J.B.*, Hagström, E., Brené, S., Mourier, A., Coppotelli, G., Freyer, C., et al. (2013). Germline mito- chondrial DNA mutations aggravate ageing and can impair brain development. Nature, 501(7467): 412-415. LINK
Freyer, C., Cree, L.M., Mourier, A., Stewart, J.B., Koolmeister, C., Milenkovic, D., et al. (2012). Variation in germline mtDNA heteroplasmy is determined prenatally but modified during subsequent transmission. Nature Genetics, 44(11): 1282–1285. LINK
Ruzzenente, B., Metodiev, M.D., Wredenberg, A., Bratic, A., Park, C.B., Cámara, Y., et al. (2012). LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. The EMBO Journal, 31(2): 443–456. LINK
Ameur, A.*, Stewart, J.B.*, Freyer, C., Hagström, E., Ingman, M., Larsson, N.-G., & Gyllensten, U. (2011). Ultra-deep sequencing of mouse mitochondrial DNA: mutational patterns and their origins. PLoS Genetics, 7(3): e1002028. LINK
Bratic, A.*, Wredenberg, A.*, Grönke, S., Stewart, J.B., Mourier, A., Ruzzenente, B., et al. (2011). The bicoid stability factor controls polyadenylation and expression of specific mitochondrial mRNAs in Drosophila melanogaster. PLoS Genetics, 7(10): e1002324. LINK
Freyer, C., Park, C.B., Ekstrand, M., Shi, Y., Khvorostova, J., Wibom, R., et al. (2010). Maintenance of respiratory chain function in mouse hearts with severely impaired mtDNA transcription. Nucleic Acids Research, 38(19): 6577–6588. LINK
Aydin, J., Andersson, D.C., Hänninen, S. L., Wredenberg, A., Tavi, P., Park, C.B., et al. (2009). Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human Molecular Genetics, 18(2): 278–288. LINK
Edgar, D.*, Shabalina, I.G.*, Cámara, Y., Wredenberg, A., Calvaruso, M.A., Nijtmans, L., et al. (2009). Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metabolism, 10(2): 131–138. LINK
Naess, K., Freyer, C., Bruhn, H., Wibom, R., Malm, G., Nennesmo, I., et al. (2009). MtDNA mutations are a common cause of severe disease phenotypes in children with Leigh syndrome. Biochimica Et Biophysica Acta, 1787(5): 484–490. LINK
Stewart, J.B., Freyer, C., Elson, J.L., & Larsson, N.-G. (2008a). Purifying selection of mtDNA and its implications for understanding evolution and mitochondrial disease. Nature Reviews. Genetics, 9(9), 657–662. LINK
Stewart, J.B., Freyer, C., Elson, J.L., Wredenberg, A., Cansu, Z., Trifunovic, A., & Larsson, N.-G. (2008b). Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biology, 6(1): e10. LINK
Freyer, C., & Larsson, N.-G. (2007). Is energy deficiency good in moderation? Cell, 131(3): 448–450. LINK
Wredenberg, A., Freyer, C., Sandström, M.E., Katz, A., Wibom, R., Westerblad, H., & Larsson, N.-G. (2006). Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochemical and Biophysical Research Communications, 350(1): 202–207. LINK
Trifunovic, A., Hansson, A., Wredenberg, A., Rovio, A.T., Dufour, E., Khvorostov, I., et al. (2005). Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production., 102(50): 17993–17998. LINK
Trifunovic, A., Wredenberg, A., Falkenberg, M., Spelbrink, J.N., Rovio, A.T., Bruder, C.E., et al. (2004). Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature, 429(6990): 417–423. LINK
Wredenberg, A., Wibom, R., Wilhelmsson, H., Graff, C., Wiener, H.H., Burden, S.J., et al. (2002). Increased mitochondrial mass in mitochondrial myopathy mice. Proceedings of the National Academy of Sciences of the United States of America, 99(23), 15066–15071. LINK
Graff, C., Wredenberg, A., Silva, J.P., Bui, T.H., Borg, K., & Larsson, N.-G. (2000). Complex genetic counselling and prenatal analysis in a woman with external ophthalmoplegia and deleted mtDNA. Prenatal Diagnosis, 20(5): 426–431.
Tollbäck, A., Eriksson, S., Wredenberg, A., Jenner, G., Vargas, R., Borg, K., & Ansved, T. (1999). Effects of high resistance training in patients with myotonic dystrophy. Scandinavian Journal of Rehabilitation Medicine, 31(1): 9–16.
Video
Support
The Wredenberglab would like to thank the following for their support:




Contact
Division of Molecular Metabolism
Dept. of Medical Biochemistry & Biophysics
Solnavägen 9 | 171 65 Stockholm | Sweden
